Bioengineering Life-Altering Gene & Cell Therapies
Expression Therapeutics is a clinical-stage biotechnology company pioneering a curative gene therapy for hemophilia A and best-in-class cell therapies for neuroendocrine tumors (NETs). Our work is powered by proprietary AI-based technological breakthroughs and an integrated 43,000 sq ft GMP manufacturing facility.
Expression Therapeutics is a clinical-stage biotechnology company pioneering a curative gene therapy for hemophilia A and best-in-class cell therapies for neuroendocrine tumors (NETs). Our work is powered by proprietary AI-based technological breakthroughs and an integrated 43,000 sq ft GMP manufacturing facility.

SSTR2 for NETs
ET3 for Hemophilia A
Cutting-Edge Science Designed for Impact
ET3 for Hemophilia A
ET3 is a next-generation, lentiviral gene therapy for people living with hemophilia A. By engineering a patient’s own blood stem cells with our optimized vector, ET3 aims to deliver sustained FVIII levels and long-lasting bleed protection. Early Phase 1 trial results show consistent FVIII expression, elimination of spontaneous bleeds, and a strong safety profile.
SSTR2 & PTK7 for NETs
ADEPT-STAR-NET is a first-in-class somatostatin receptor 2 (SSTR2)-targeting cell and gene therapy designed for the treatment of neuroendocrine tumors with the potential for label expansion to other SSTR2+ cancers. The product consists of autologous gamma delta T cells expanded ex vivo and engineered using a recombinant lentiviral vector to secrete a bispecific T cell engager (TCE) that targets CD3 and SSTR2. By combining the innate cytotoxicity of gamma delta T cells with localized TCE-mediated recruitment of additional T cells, the ADEPT-STAR platform is designed to achieve potent, tumor-selective immune activation while minimizing systemic toxicity.
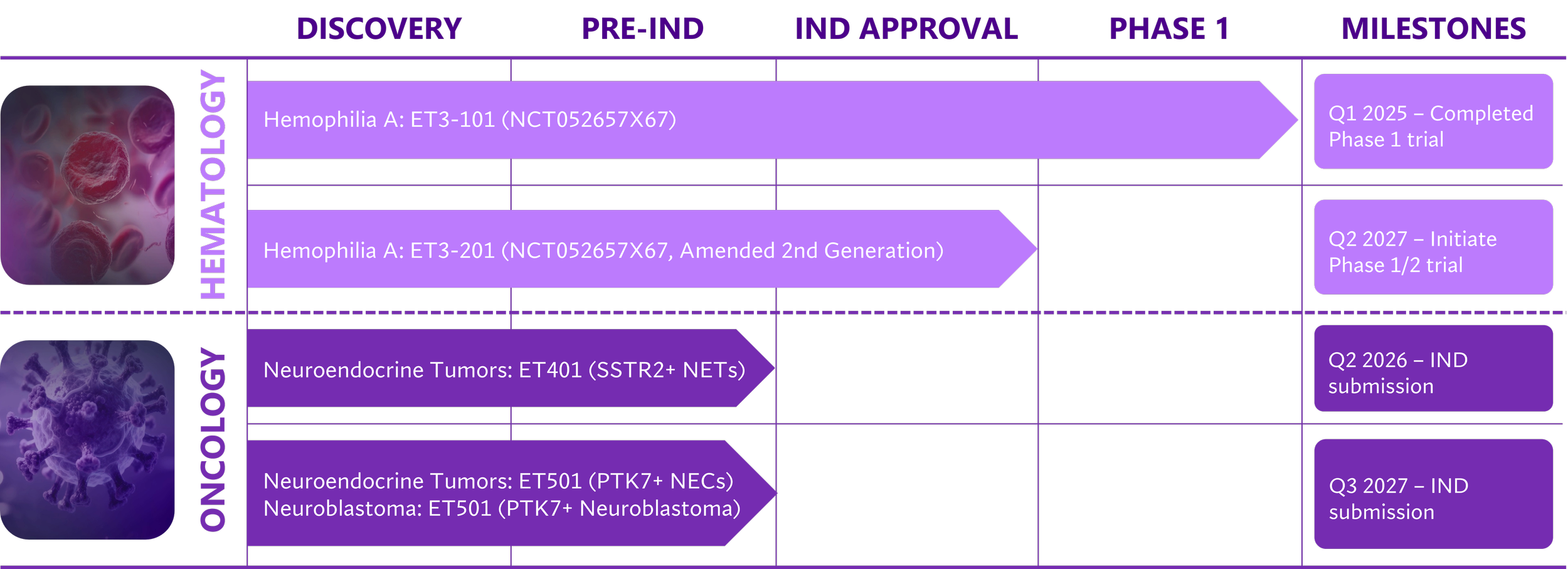
Therapeutic Pipeline
Control from
Discovery to Delivery
Control from Discovery to Delivery
Our 43,000 sq ft manufacturing facility in Cincinnati, Ohio is equipped with advanced modular cleanrooms, GLP labs, and an experienced team led by industry pioneer William Swaney giving us full control of lentiviral and cell therapy manufacturing. Powered by our proprietary eCO and LENTeT technologies, we accelerate development from IND through late-stage readiness and beyond, positioning programs for future success.