Expression Therapeutics is developing a best-in-class gene therapy for Hemophilia A.
-
Hemophilia A is an inherited bleeding disorder caused by a deficiency of clotting Factor VIII (FVIII), leading to uncontrolled bleeding and life-threatening complications without treatment.
-
Patients with severe hemophilia A face frequent bleeding episodes, significant morbidity, and lifelong dependence on intensive FVIII infusion regimens to prevent joint damage and life-threatening bleeding.
-
Standard care requires intravenous FVIII infusions several times each week, placing a heavy treatment burden on patients and still failing to provide durable or curative outcomes.
-
Managing hemophilia A prophylactically in the U.S. costs more than $650,000 per patient per year, making it one of the most expensive lifelong treatments for any genetic disorder.
-
A durable, curative gene therapy has the potential to eliminate the need for frequent FVIII infusions, dramatically reduce lifetime healthcare costs, and significantly improve the quality of life for patients.
Our Lead Product for Hemophilia A
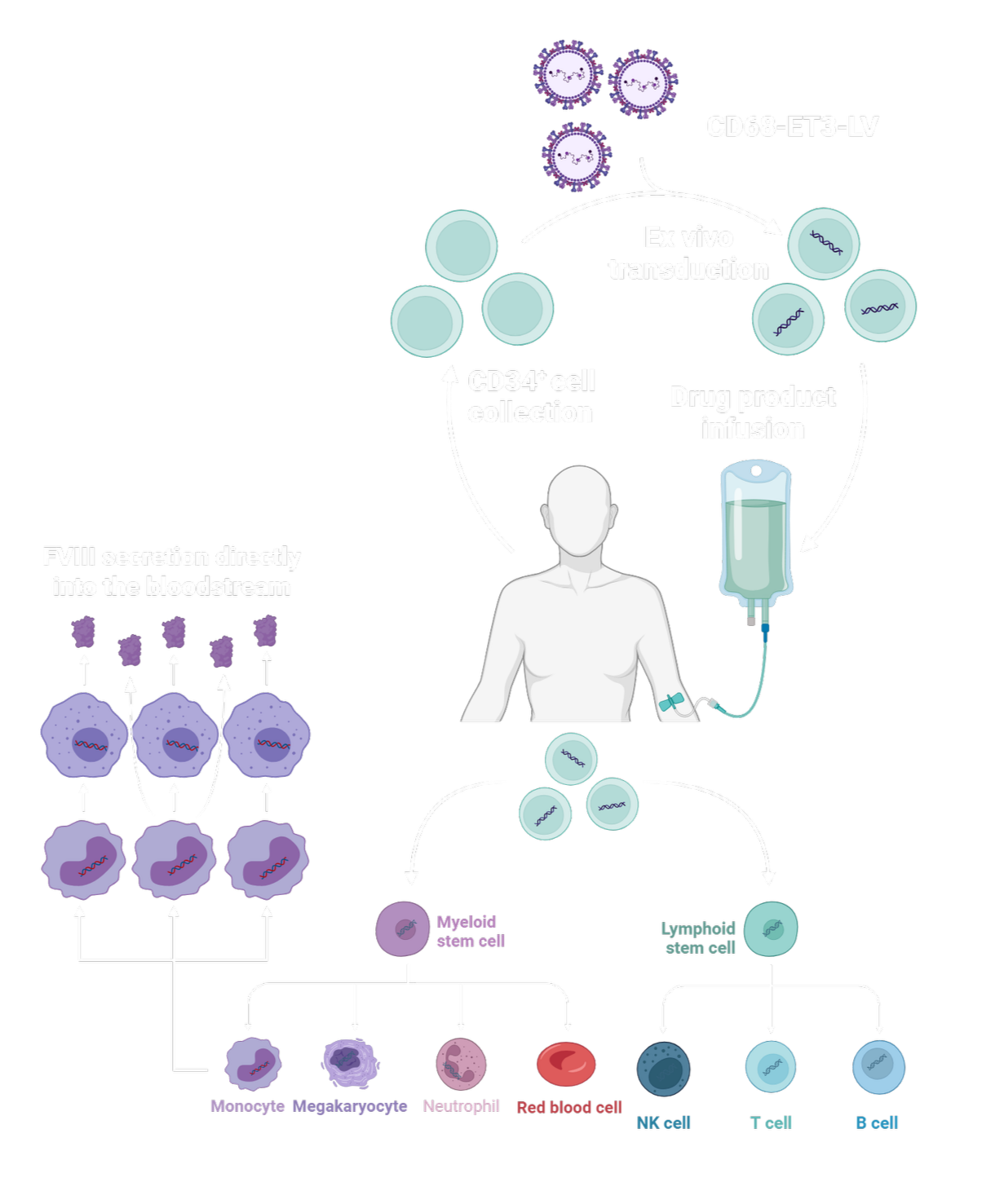
Ex vivo hematopoietic stem cell (HSC) LV gene therapy that works derived from the patient (“autologous”) cells
-
White blood cells are harvested from the blood of a patient through a process called apheresis.
-
In the laboratory, hematopoietic (blood) stem and progenitor cells are enriched through a process termed CD34+ selection.
-
The CD34+ cells are genetically modified (transduced) using our proprietary LV-FVIII vector and then transplanted back into the patient via a simple peripheral vein infusion.
-
The CD68-ET3-LV CD34+ cell product functions by engrafting back into the stem cell compartment within the bone marrow and resuming its normal function of blood cell production.
-
Following the genetic modification procedure, millions of daughter blood cells now provide a continuous supply of functional FVIII to the bloodstream.

Phase 1 Clinical Results
We have completed a Phase 1 clinical trial. The clinical trial recruited five participants 22 to 41 years of age with severe hemophilia A. Autologous hematopoietic stem cells (HSCs) were transduced with CD68-ET3-LV — a lentiviral vector including a novel FVIII transgene (ET3) with a myeloid-directed CD68 promoter.
-
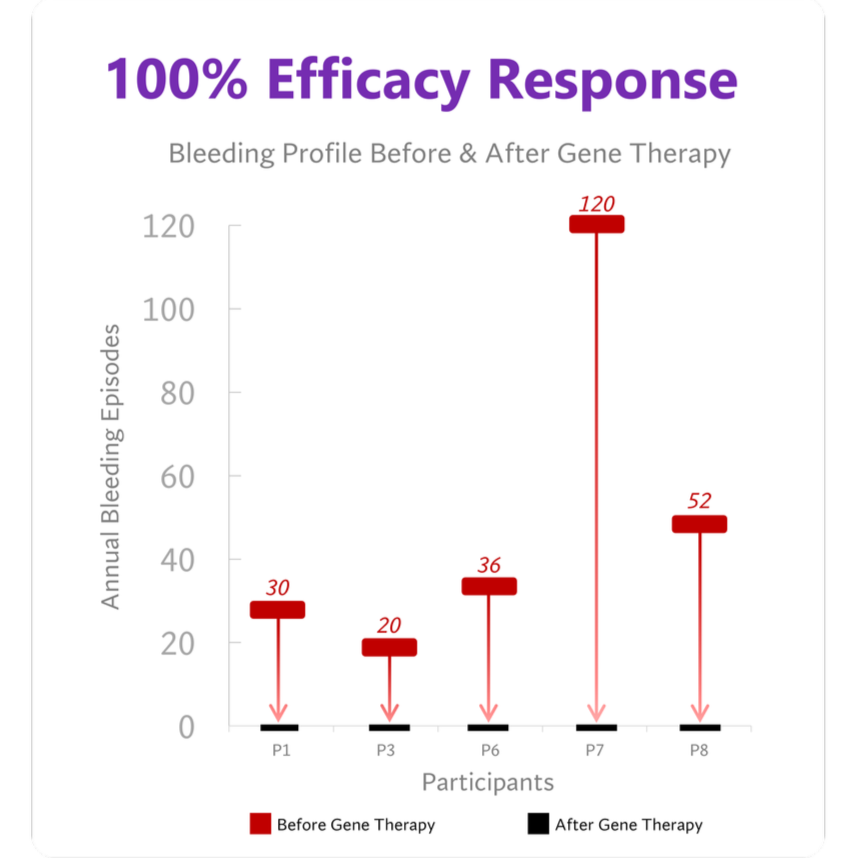
Before gene therapy, all participants reported annualized bleeding rates of at least 20 events. No spontaneous bleeding events occurred in any participant on study.
-
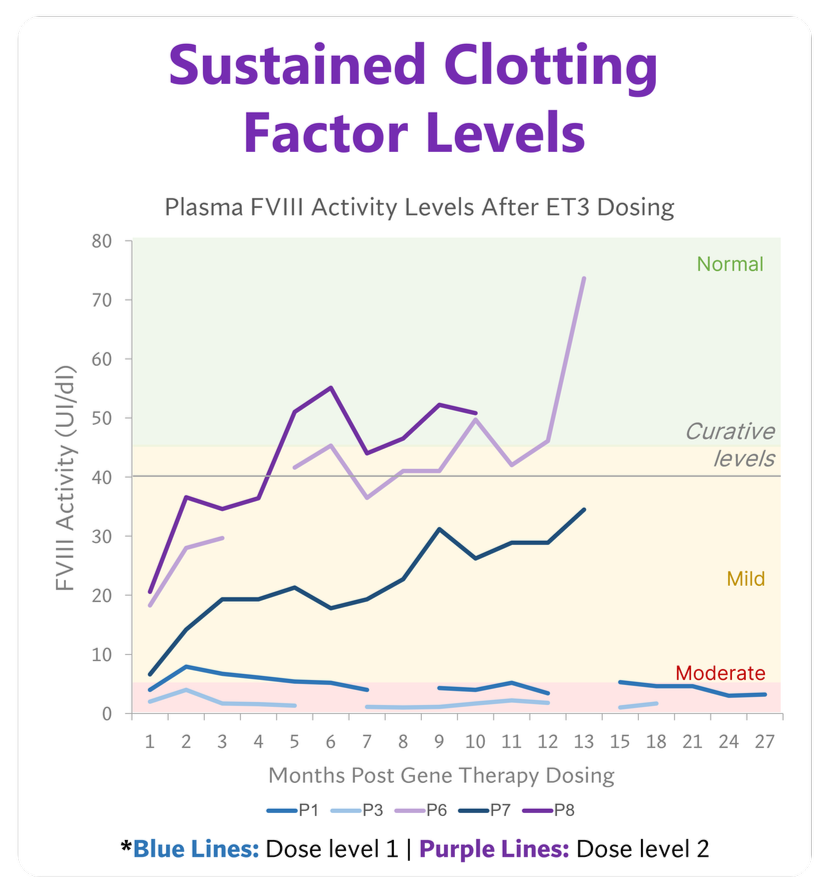
Measured FVIII levels in all five participants increased from undetectable to levels predicted to produce therapeutic efficacy. The median follow-up was 14 months (range, 9 to 27 months).
-
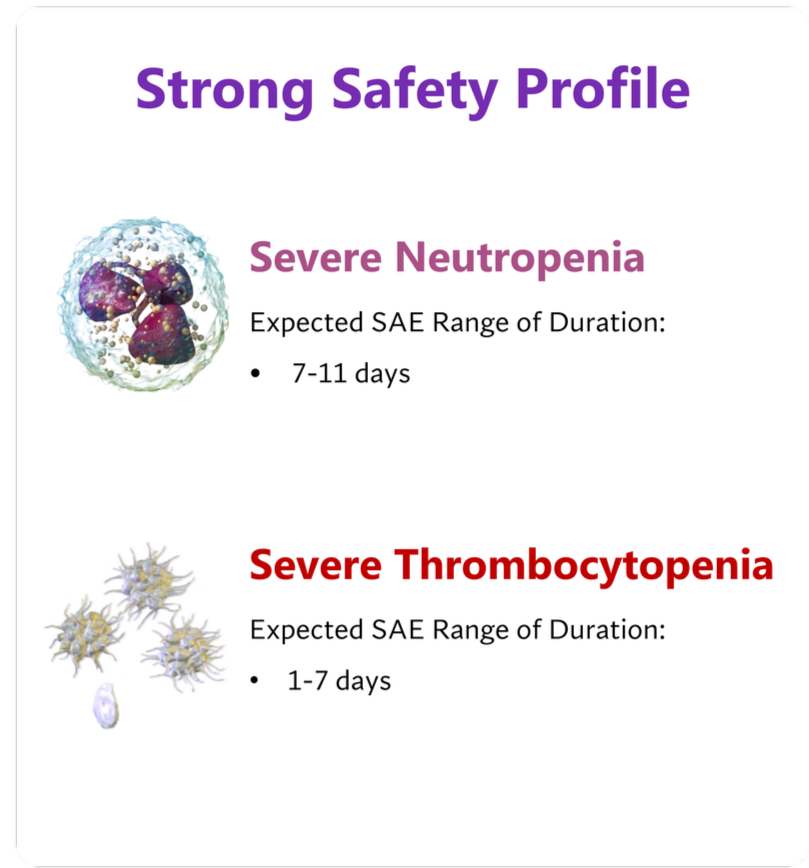
No adverse events greater than grade 2 occurred in any participant, beyond those expected with neutropenia and thrombocytopenia.
Inhibitors to FVIII did not develop in any participant after drug-product infusion. Integration-site analysis performed at 4 to 22 months after drug-product infusion showed no safety concerns.
The results of this Phase 1 clinical trial validate a new approach to gene therapy for hemophilia A that can provide lifelong durability of expression. The candidate CD68-ET3-LV-CD34+ gene therapy product overcomes the limitations of the AAV gene therapy approaches to hemophilia A, including lack of durable expression, patient ineligibility on the basis of age, and preexisting anti-AAV antibody-related conditions. Our lentiviral gene therapy is currently the only approach that offers the possibility of a permanent cure for hemophilia A and provides an opportunity to reach both pediatric and adult populations.