Expression Therapeutics is developing a Best-in-Class gene therapy for neuroendocrine tumors.
Expression Therapeutics is developing a Best-in-Class gene therapy for neuroendocrine tumors.
Understanding Neuroendocrine
Tumors (NETs)
The neuroendocrine system is a complex network of specialized cells that release bioactive agents into the bloodstream. NETs can develop in various organs throughout the body but most commonly in the gastrointestinal tract and pancreas (gastroenteropancreatic or GEP-NETs).
249,000
individuals in the U.S. are living with NETs.
12,000+
U.S. individuals are diagnosed with a NET per year.
30+
years of steadily rising NET incidence (U.S. and global).
Complex Biology, Limited Options
NETs treatment
options depend on…
Tumor size
Tumor location
Tumor grade
Malignant spread
NETs treatment
approaches include…
Surgical removal (if possible)
Targeted drug therapy
Chemotherapy
Peptide receptor radionuclide
therapy (PRRT)Liver-directed therapies
(in some cases)
The majority of NETs, and specifically slow growing (i.e. low-grade) well-differentiated GEP-NETs, express the surface protein, somatostatin receptor type 2 (SSTR2), which is the basis for both diagnosis, imaging (including monitoring for progression), and symptomatic therapy. Other than rare cases where the primary tumor has not spread and is surgically accessible, there are no curative therapies for NETs.
Surgical removal
Targeted drug therapy
Chemotherapy
Peptide receptor radionuclide therapy
Liver-directed therapies
Existing NET Therapies Control
Symptoms—Not Tumors
Existing NET Therapies Control Symptoms—Not Tumors
The primary approved drugs for NETs are somatostatin analogs, SSA-based PRRT, and a tryptophan hydroxylase inhibitor that blocks serotonin biosynthesis. These drugs can help alleviate symptoms associated with the secretion of bioactive substances, however, they demonstrate little to no effect on reducing tumor burden or generating partial or complete response (PR/CR).
-
Octreotide® (a synthetic SSA) alone demonstrates only 4% ORR (all PR, no CR).
-
Lutathera® (PRRT), demonstrates ~75% reduction in the risk of death or disease progression, but only 13% overall response rate (ORR; PR+CR) in individuals with low-grade, well differentiated NETs whose disease had progressed on SSAs.
PRRT requires expensive radioprotective infrastructure and is inherently radiotoxic (93% adverse events; 2-3% secondary malignancies to date, and possible infertility).
-
Not sufficient as a monotherapy for NETs because it primarily manages symptoms associated with excess serotonin production, not the underlying tumor growth or progression.
Designed to Eliminate Tumors,
Not Just Manage Them
Designed to Eliminate Tumors, Not Just Manage Them
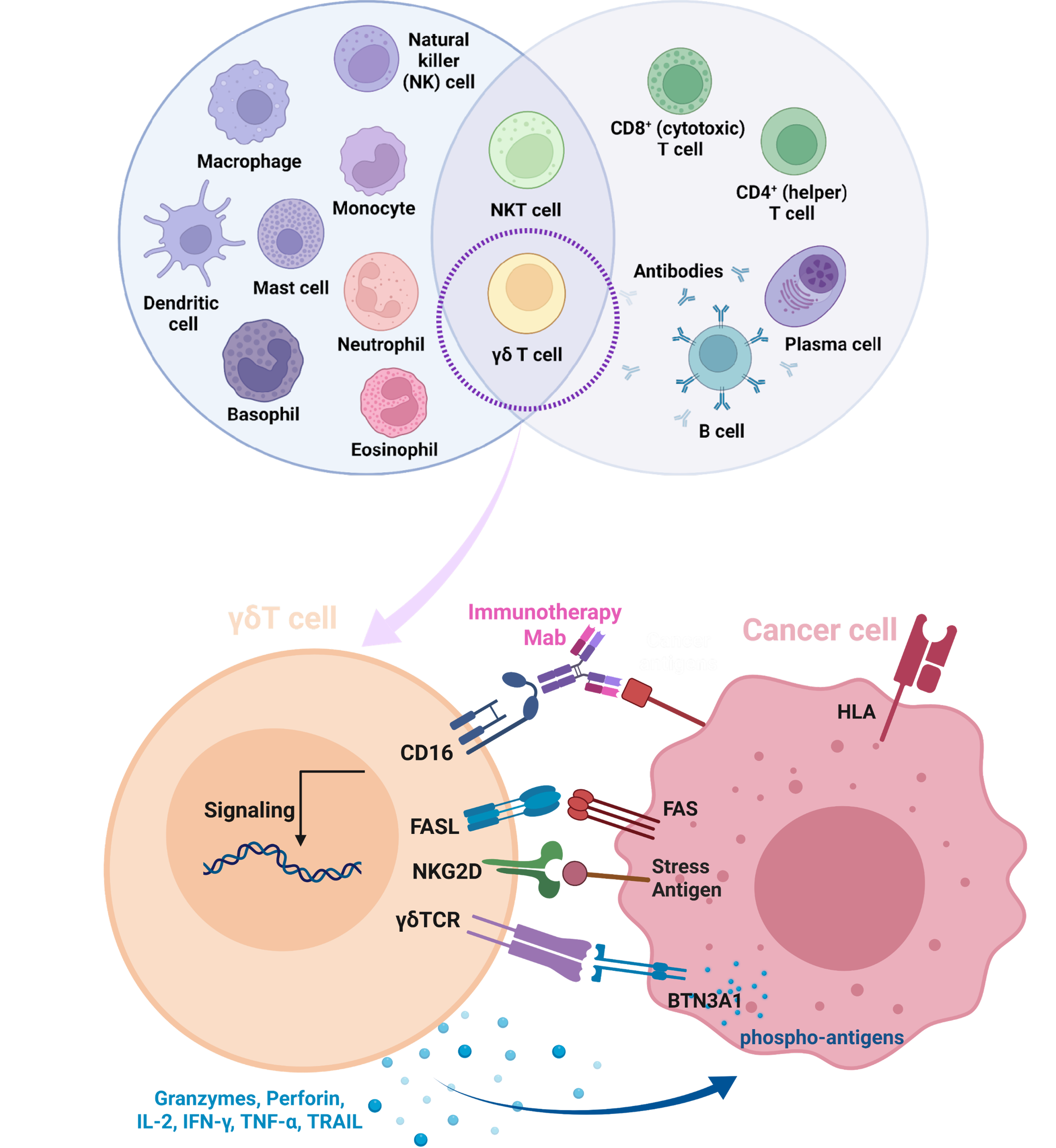
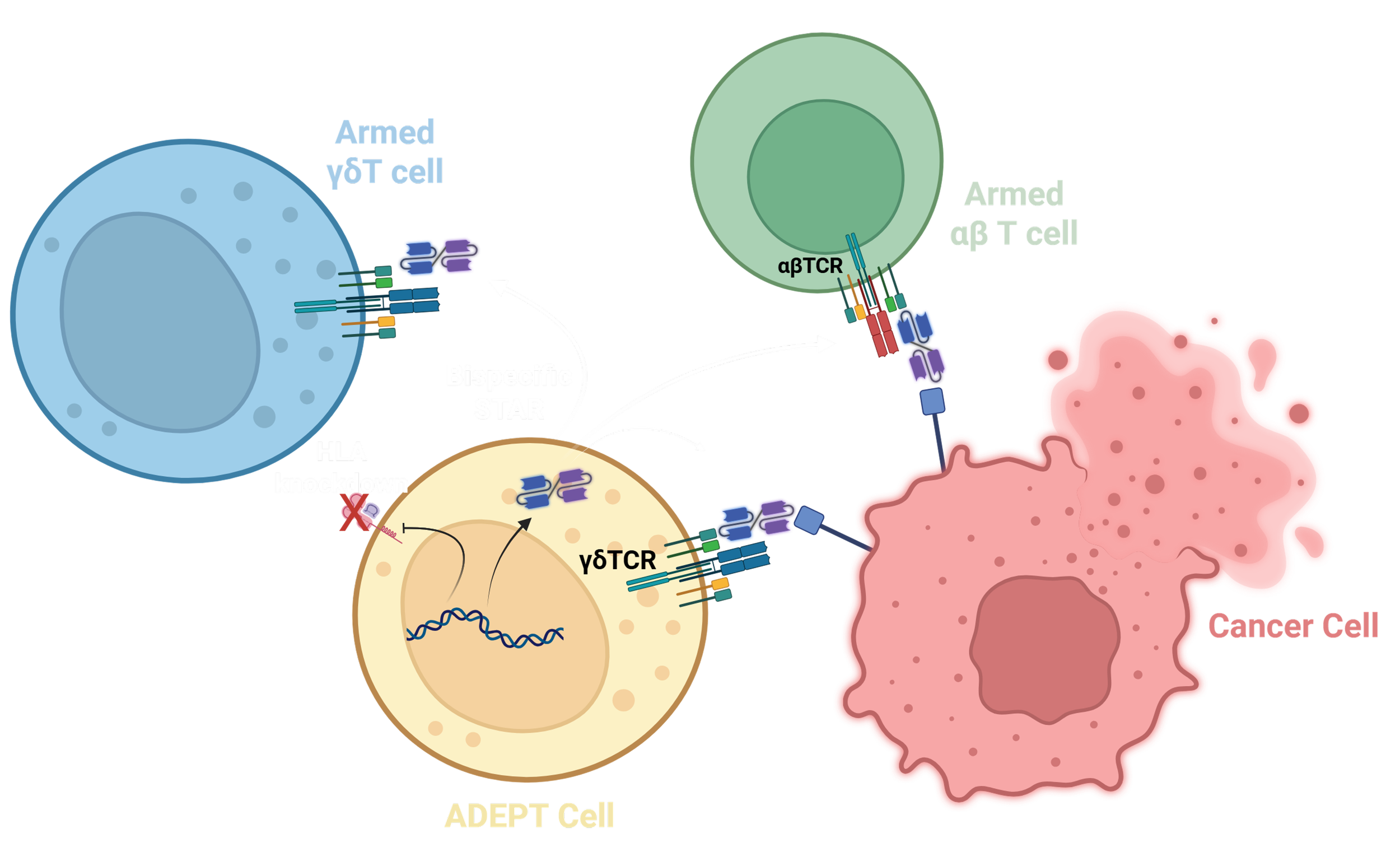
We are developing a best-in-class cell therapy utilizing autologous (i.e. patient) derived, expanded, and programmed γδT cells (‘ADEPT’) to deliver a secreted T cell engager (TCE) targeting SSTR2 as the surface tumor antigen and capable of engaging all CD3+ cells in the cell product as well as the patient’s endogenous T cells.
ADEPT cells are programmed using the proprietary LENTeT gene transfer system to express Secreted T cell ActuatoRs (‘STARs’), which elevate the specificity and potency of the cell product. ‘STAR’ is an umbrella term to describe the proteins genetically engineered to be expressed from ADEPT cells. The ADEPT-STAR mechanism of action does not rely on rapid cell division as standard chemotherapy requires, or high mutational burden and/or morphology changes which are common targets of immunotherapies. Instead, ADEPT-STAR can be programmed to kill any cell (dividing or resting) displaying a threshold level of a target surface antigen.
Our lead candidate, a uniquely engineered CD3ε x SSTR2 bispecific TCE, designated STAR-NET-1 is the prime actuator in the ADEPT-STAR-NET cellular machine. In preclinical studies, STAR-NET-1 confers potent cytotoxic activity to unmodified γδT cells against SSTR2+ target cells with an effective concentration 50% (EC50) in the low femtomolar range (159 ± 75 fM).
One of the challenges in NET drug discovery is the lack of representative animal models of the disease. To address this, a state-of-the-art non-animal method (NAM) incorporating GEP-NET patient-derived spheroid cultures has been employed for ADEPT-STAR-NET-1 development. In this model, ADEPT-STAR-NET-1 displays potent cytotoxic activity resulting in uniform spheroid eradication at low effector to target cell ratios.
By focusing initially on SSTR2, a well-validated imaging and therapeutic target in NETs, as well as several other prevalent cancers (e.g. small cell lung cancer, meningioma and certain gliomas, colorectal cancer, hepatocellular carcinoma, and urothelial carcinoma), Expression Therapeutics aims to create a highly specific, safe and effective immuno-oncology therapy that can complement or replace existing treatments like somatostatin analogs, peptide receptor radionuclide therapy, and chemotherapy. Expression Therapeutics is also exploring additional TCE STAR targets that will expand the oncology market to include more aggressive neuroendocrine carcinomas (NECs), neuroblastoma and other deadly cancers.